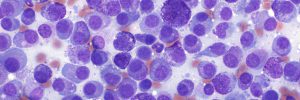
Cytology sample advice

Important note: Our definition of a plain tube is one that has no additives e.g. microcentrifuge/Epenndorf tubes. Please do not use blood serum tubes as these contain fine powder and/or powder coated plastic beads. When the powder is sedimented in-lab, it can mask the cellular component of the sample. This is a particular problem with CSF and aqueocentesis samples due to their low cellularity.
Sample Advice - General Considerations
Please complete the submission form including the animal’s full signalment, a pertinent clinical history and your clinical impression/differential diagnoses. This information will ensure correct interpretation of the pathologist’s findings.
All specimens should be labelled with at least the name of the animal and the owner so that identification of the specimens can be verified.
Formalin should never be added to non-histopathology specimens (please see the CSF section for the exception). Formalin and even formalin fumes fix the cell membranes and this reduces the ability of our cytological stains to penetrate, with consequent loss of staining detail. We highly recommend histopathology specimens be sent separately from other specimens to avoid the inadvertent contamination of these other specimens with formalin fumes.
In all cases, keeping the smears as thin as possible and rapid air-drying of the smears (preferably with the use of a hair dryer) is essential to avoid so-called 'drying artefact'. Also sometimes referred to as shrinkage artefact, this is a particularly important problem in fluid or blood-rich samples.
Sample Advice - Specific Considerations
In all cases, keeping the smears as thin as possible and rapid air-drying of the smears (preferably with the use of a hair dryer) is essential to avoid so-called 'drying artefact'. This is particularly important in fluid or blood-heavy samples.
Solid tissue samples
(e.g. solid tumours, surface scrapings of mucous membranes/skin)
Please submit air-dried, unstained smears on frosted slides.
Fluid-filled solid tissue samples
(e.g. abscesses, cysts, seromas, solid tumours with cystic/fluid centres)
Please submit air-dried, direct, unstained smears of the liquid component together with liquid in EDTA for cytology. If microbiology is required, additional fluid should be placed in a plain sterile tube as EDTA is unsuitable for culture. When anatomically achievable, please also send air-dried, unstained smears of the solid component of the lesion.
Thoracic, abdominal, pericardial and joint fluids
Please submit:
- Fluid in EDTA (need to prevent clotting for a reliable nucleated cell count) – ensure good mixing and fill the EDTA tube to the fill-line. For very viscous joint fluid, we recognise good mixing can be difficult
- Two air-dried, unstained, direct smears of the fluid
- If you have a centrifuge available, any excess fluid can be spun down, the supernatant discarded, and air-dried, unstained sediment smears prepared. Please mark them as ‘sediment’.
Note: EDTA samples are not suitable for bacterial culture. Either a swab of the fluid or fluid in a sterile plain tube can be submitted for microbiology.
Flush fluid
(e.g. bronchoalveolar lavage, nasal flush, prostatic wash)
Please submit:
- Fluid in plain or EDTA (because the cells are diluted by the large volume of flush fluid, clotting is not really an issue and so the use of EDTA is not imperative in this case)
- Air-dried, unstained, direct smears of the fluid
- If you have a centrifuge available, any excess fluid can be spun down, the supernatant discarded, and air-dried, unstained sediment smears prepared. Please mark them as ‘sediment’.
Cerebrospinal fluid (CSF)
Due to the normally extremely low cellularity of CSF fluids it is not usually worthwhile preparing direct smears for submission. This is one special situation in which we would like you to send a formalinised sample.
Please submit:
- Fluid in plain tube (e.g. microcentrifuge tube/Eppendorf tube)
- Fluid in plain tube + 1 drop of 10% formalin
Please mark the tube containing the formalin (this is important – we perform different tests on the different tubes). Do not submit CSF in serum tubes that contain mixing beads (even if the beads have been removed) or serum tubes that are coated with a clotting agent powder (e.g. serum gel tubes, Zn-tubes) - see important note below.
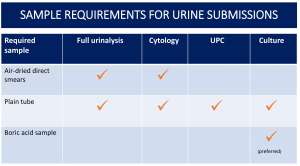
Urine for cytology +/- urinalysis
Please submit:
- Urine in a plain tube
- Air-dried, unstained, direct smears
- If you have a centrifuge available, any excess fluid can be spun down, the supernatant discarded and air-dried, unstained sediment smears prepared. Please mark them ‘sediment’.
Aqueocentesis samples
It may be worth a quick word with the pathologist prior to taking the sample as the methods for submitting aqueous somewhat depend of what the suspected diagnosis is.
If your practice has a cytospin machine on site (please note that this is not the same as a routine centrifuge machine, see image below), submission of unstained, air-dried cytospin slides is ideal. Direct smears are rarely helpful unless there is a significant amount of flare or fibrin in the anterior chamber in which case, a small drop can be used to make one direct smear that should be quickly air dried (a hair dryer works well) and submitted unstained. The remaining fluid can then be submitted in a plain tube (e.g. microcentrifuge tube/Eppendorf tube) so that we can perform a cytospin preparation for cytology. A normal EDTA tube tends to have too high a concentration of EDTA for the small amount of aqueous submitted so unless a paediatric tube is available, a plain tube is preferable. As cell deterioration is problematic in these samples, samples are best sent by courier or special next day delivery.

Important note: Our definition of a plain tube is one that has no additives e.g. microcentrifuge/Epenndorf tubes. Please do not use blood serum tubes as these contain fine powder and/or powder coated plastic beads. When the powder is sedimented in-lab, it can mask the cellular component of the sample. This is a particular problem with CSF and aqueocentesis samples due to their low cellularity. If you must use blood serum tubes, please wash them beforehand and ensure they are dry before use.
If you have any questions or if you are concerned about the quality of your cytology samples and don't know how to fix it, please just give us a call and speak to one of the pathologists who will be more than happy to try and help.